Can Motif Bio really surge 400%?
5th April 2017 13:14
by David Brenchley from interactive investor
Share on
When was the last time you were told there was an investment that could make five times your money? Well, broker finnCap thinks , the company behind a late-stage antibiotic called iclaprim, could do the job.
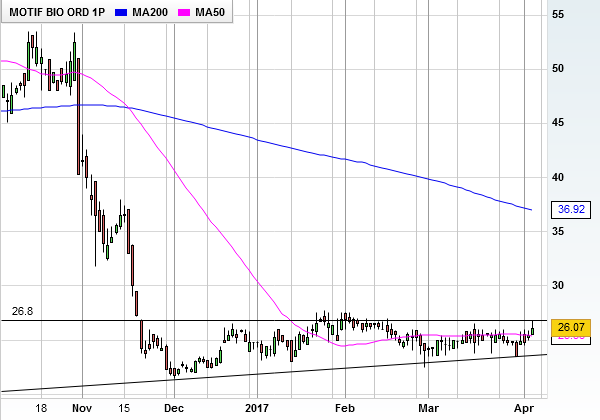
Initiating coverage of the AIM-listed shares, analyst Alex Pye thinks they could be worth 125p. After funding concerns last autumn dumped the shares from 53p to 21p – just a penny above the 2015 IPO price of 20p - that bullish target implies potential upside of a colossal 400%.
However, don't put that deposit down on the new Ferrari just yet! Pye's objective is the product of a 13-year view, reflecting a net present value of £245 million using a risk-adjusted discounted cash flow model.
So, it could be a long road, and there's a bucketload of risk here, but there are also plenty of potential catalyst events to get the shares shifting in the right direction. And finnCap believes a re-rating, at least, could begin soon.
Watch for a positive phase III REVIVE data readout in the second quarter of 2017, says Pye. This is the clinical trial investigating the safety and efficacy of iclaprim to treat patients with Acquired Bacterial Skin and Skin Structures Infection (ABSSSI).
He points out that shares in Nasdaq–listed Achaogen more than tripled overnight last December following positive phase III results for its gram-negative candidate plazomicin, another drug which attacks super bugs.
But Motif Bio is short of cash and needs another $10 million to complete its second phase III trial, REVIVE-2. FinnCap believes that a positive REVIVE-1 readout should attract potential backers and make an equity fundraising much easier.
"Motif worked closely with the US Food and Drug Administration (FDA) in designing these pivotal trials and if primary endpoints are reached successfully, we expect the shares to be re-rated," writes Pye.
There could also be upside if Motif Bio agrees a partnering licence agreement with a "notable and respected pharmaceutical company for upfront payments and reduced development costs", Pye says. Grant funding from numerous possible sources would be a further catalyst.
Comparable antimicrobials have already gained approval, he explains: "Daptomycin [achieved] more than $1 billion global sales, all with similar clinical trial designs."
Motif Bio shares are already inching higher as finnCap's argument is very persuasive, but small biotechs like this are incredibly high risk.
Confidence took a huge hit late last year funding issues arose and, although money was forthcoming, the company needs more, potentially up to $42 million to complete Phase III trials in Hospital-Acquired Bacterial Pneumonia (HABP) for iclaprim. Thankfully, REVIVE-1 results are expected before net cash falls below zero.
There's also a chance that these results will be disappointing. But here, too, Pye thinks risk is mitigated by the "overwhelmingly positive" historical Phase II and III data that support the safety and efficacy of iclaprim.
As we wrote in June 2015, one to watch.
This article is for information and discussion purposes only and does not form a recommendation to invest or otherwise. The value of an investment may fall. The investments referred to in this article may not be suitable for all investors, and if in doubt, an investor should seek advice from a qualified investment adviser.